Your Electron configuration of calcium in ground state images are ready. Electron configuration of calcium in ground state are a topic that is being searched for and liked by netizens today. You can Find and Download the Electron configuration of calcium in ground state files here. Get all royalty-free vectors.
If you’re searching for electron configuration of calcium in ground state images information connected with to the electron configuration of calcium in ground state topic, you have come to the right site. Our site frequently gives you suggestions for refferencing the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
Electron Configuration Of Calcium In Ground State. Electron configuration of Calcium is Ar 4s2. The orbital approximation allows us to express the electronic structure of an atom by reporting its configuration the list of occupied orbitals. Electron as occupying its own orbital. 3d and 4s orbitals.
 Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes From docbrown.info
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes From docbrown.info
Calcium Complete Electron Configuration. The ground state of Ar atom also has electron configuration of 2 8 8. The ground state electron configuration of ground state gaseous neutral calcium is Ar. For instance the ground state electronic configuration of calcium Z20 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Written By Cambell Crins1943 Monday November 22 2021 Add Comment Edit In the last section we determined that the members of the chemical families vertical columns on the periodic table all have the same electron configuration in their outermost energy levels. 1s2 2s2 2p6 3s2 3p6 4s2 or simply Ca.
Electron configuration of Calcium is Ar 4s2.
1s2 2s2 2p6 3s2 3p6 4s2. Calcium atoms have 20 electrons and the shell structure is 2882. 1s2 2s2 2p6 3s2 3p6 4 s2. Electronic configuration shows the arrangement of electrons around the nucleus. Electron as occupying its own orbital. Hence the electron configuration for Ca 2 is 1s 22s 22p 63s 23p 6.
 Source: wikihow.com
Source: wikihow.com
Calcium Complete Electron Configuration. Calcium Complete Electron Configuration. How do you write calcium chloride formula. The p orbital can hold up to six electrons. Calcium atoms have 20 electrons and the shell structure is 28.
 Source: slidetodoc.com
Source: slidetodoc.com
Hence the electron configuration for Ca 2 is 1s 22s 22p 63s 23p 6. GROUND STATE CONFIGURATION for first 6 elements. A calcium 2 ion. The electron configuration of calcium ion Ca2 is 2 8 8. 4s 2 and the term symbol is 1 S 0.
 Source: wikihow.com
Source: wikihow.com
Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The ground state electron configuration for calcium is eq1s22s22p63s23p64s2 eq. Electron as occupying its own orbital. Hence the electron configuration for Ca 2 is 1s 22s 22p 63s 23p 6. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structureThe chemical symbol for Calcium is Ca.
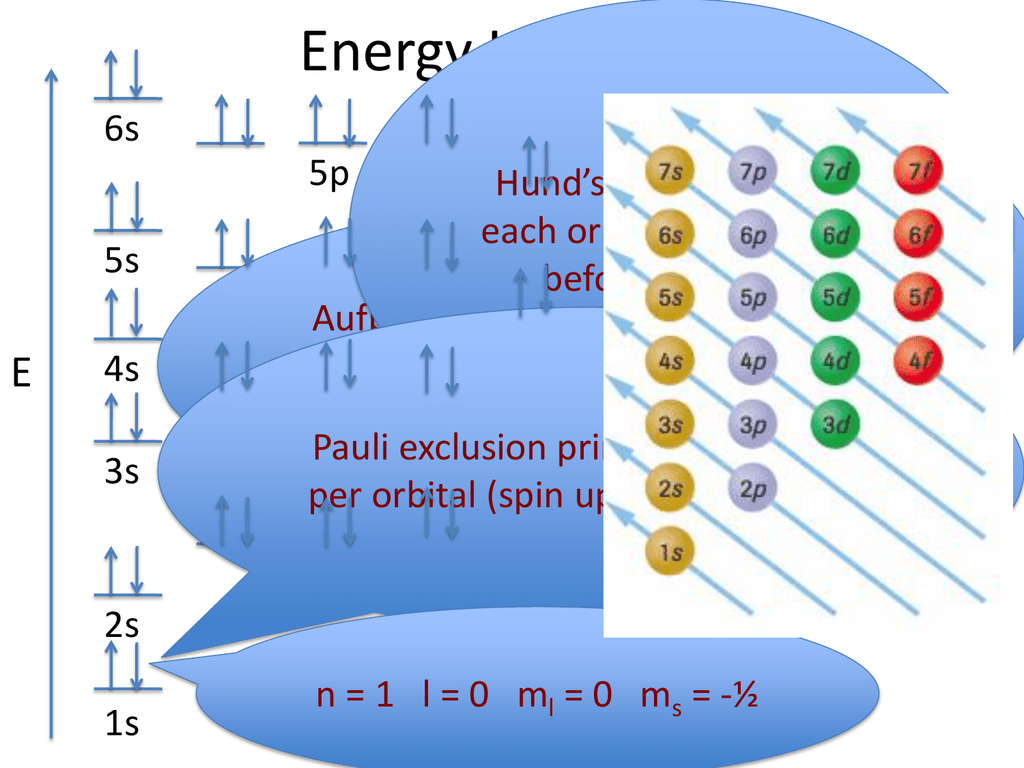 Source: studylib.net
Source: studylib.net
Written By Cambell Crins1943 Monday November 22 2021 Add Comment Edit In the last section we determined that the members of the chemical families vertical columns on the periodic table all have the same electron configuration in their outermost energy levels. The ground state electron configuration of ground state gaseous neutral calcium is Ar. 4s 2 and the term symbol is 1 S 0. Ca has no charge which means that no electrons are removed or added in the atom. 4s2 and the term symbol is 1S0.
 Source: clutchprep.com
Source: clutchprep.com
The calcium ion Ca 2 however has two electrons less. A neutral calcium atom also has 20 electrons. The calcium ion Ca 2 however has two electrons less. Therefore the phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Electron as occupying its own orbital.
 Source: youtube.com
Source: youtube.com
The answer is 1 Ar. Electronic Configurations of Cations and Anions For instance the ground state electronic configuration of calcium Z20 is 1s 22s 22p 63s 23p 64s 2. Possible oxidation states are 2. The electron configuration of a neutral calcium atom is 1s22s22p63s23p64s2. You can determine the ground-state electron configuration of Calcium Ca by referring to the periodic table and locating the position of Ca in the periodic table.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
The calcium ion Ca 2 however has two electrons less. Ar 4s2 Calcium has an atomic number of 20. So the answer is 1. Nevertheless check the complete configuration and other interesting facts about Calcium that most people dont know. Electronic Configurations of Cations and Anions for instance the ground state digital configuration the calcium Z20 is 1s 22s 22p 63s 23p 64s 2.
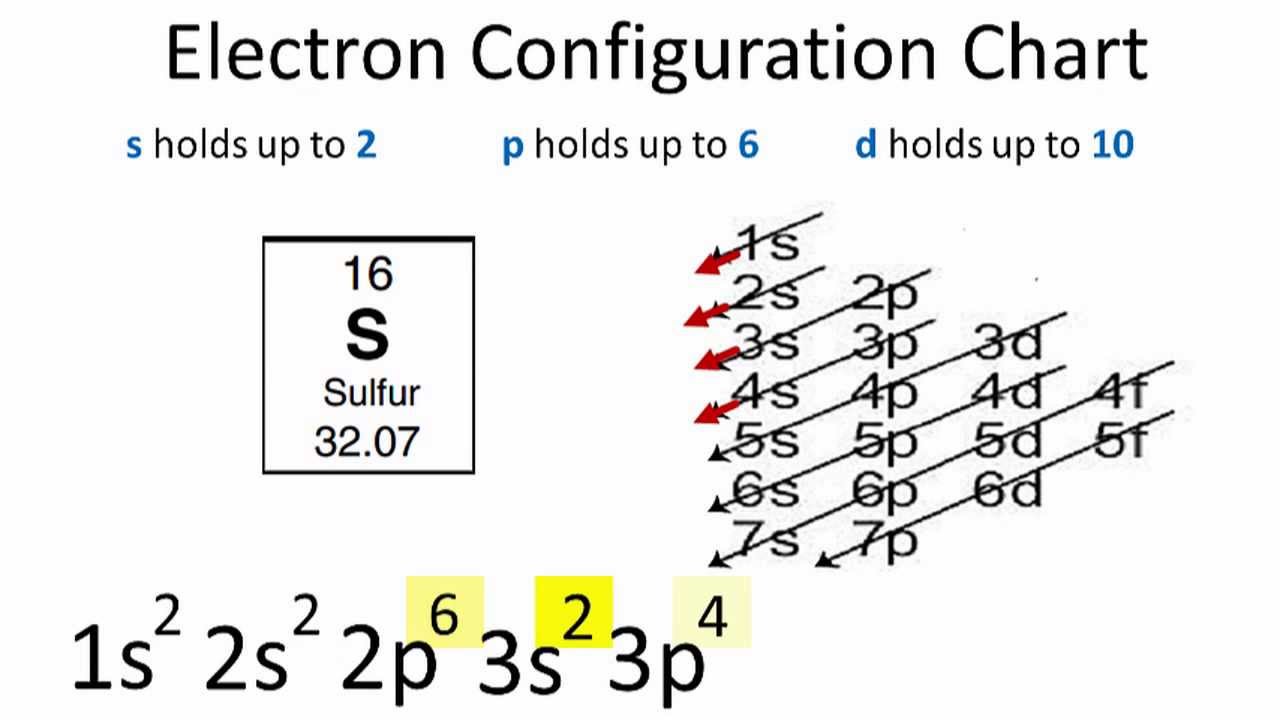 Source: periodictable.me
Source: periodictable.me
1s2 2s2 2p6 3s2 3p6 4s2. Since we need to take away two electrons we first remove electrons from the outermost shell n4. Electron as occupying its own orbital. 4s2 and the term symbol is 1S0. Hence the electron configuration for Ca 2 is 1s 2 2s 2 2p 6 3s 2 3p 6.
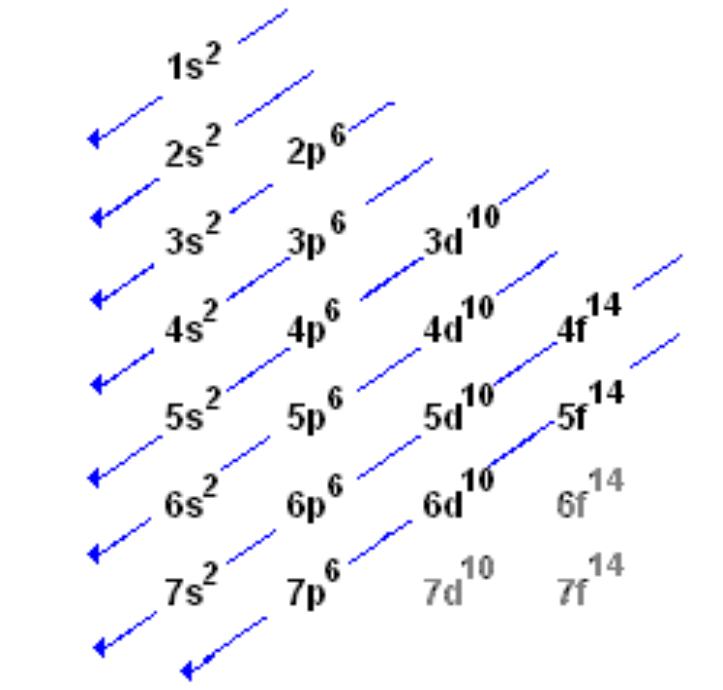 Source: chem.fsu.edu
Source: chem.fsu.edu
Schematic electronic configuration of calcium. Calcium chloride contains calcium and chloride ions. Write the ground-state electron configuration for the calcium ion. The p orbital can hold up to six electrons. Hence the electron construction for Ca 2 is 1s 22s 22p 63s 23p 6.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
Hence the electron construction for Ca 2 is 1s 22s 22p 63s 23p 6. The ground state electron configuration of ground state gaseous neutral calcium is Ar. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structureThe chemical symbol for Calcium is Ca. A calcium 2 ion. Ca has no charge which means that no electrons are removed or added in the atom.

Ar 4s2 Calcium has an atomic number of 20. So the answer is 1. You may write either the full or condensed electron configuration. 4s 2 and the term symbol is 1 S 0. The electron configuration of a neutral calcium atom is.
 Source: docbrown.info
Source: docbrown.info
Since calcium is in the fourth row and the second column of the s-block on the periodic table of elements its electron configuration ends in 4s2. 4s2 and the term symbol is 1S0. In several cases the ground state electron configurations are different from those predicted by Figure 221. The calcium ion Ca 2 however has two electrons less. How do you write calcium chloride formula.
 Source: researchgate.net
Source: researchgate.net
A calcium 2 ion. 4s2 and the term symbol is 1S0. The ground state electron configuration of ground state gaseous neutral calcium is Ar. Write the ground-state electron configuration for the calcium ion. The chemical formula of calcium chloride is CaCl2Properties Of Calcium Chloride Formula.
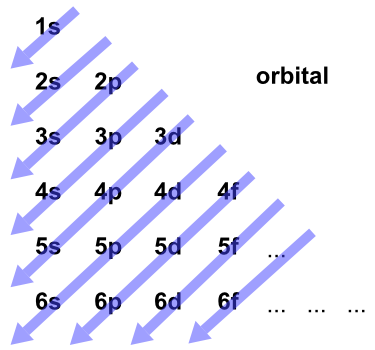 Source: chemedx.org
Source: chemedx.org
1s2 2s2 2p6 3s2 3p6 4 s2. Written By Cambell Crins1943 Monday November 22 2021 Add Comment Edit In the last section we determined that the members of the chemical families vertical columns on the periodic table all have the same electron configuration in their outermost energy levels. Calcium atoms have 20 electrons and the shell structure is 28. Write the ground-state electron configuration for the calcium ion. You can determine the ground-state electron configuration of Calcium Ca by referring to the periodic table and locating the position of Ca in the periodic table.
 Source: iperiodictable.com
Source: iperiodictable.com
Calcium chloride contains calcium and chloride ions. Electron Configuration and Oxidation States of Calcium. Electronic Configurations of Cations and Anions For instance the ground state electronic configuration of calcium Z20 is 1s 22s 22p 63s 23p 64s 2. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structureThe chemical symbol for Calcium is Ca. For instance the ground state electronic configuration of calcium Z20 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
Write the ground-state electron configuration for the calcium ion. Electron configuration of Calcium is Ar 4s2. Possible oxidation states are 2. 1s2 2s2 2p6 3s2 3p6 4 s2. The atomic number of calcium is 20.
 Source: iperiodictable.com
Source: iperiodictable.com
Electronic Configurations of Cations and Anions for instance the ground state digital configuration the calcium Z20 is 1s 22s 22p 63s 23p 64s 2. 1s2 2s2 2p6 3s2 3p6 4 s2. Calcium atoms have 20 electrons and the shell structure is 2882. The answer is 1 Ar. Possible oxidation states are 2.
 Source: webelements.com
Source: webelements.com
Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. Possible oxidation states are 2. The ground state electron configuration of ground state gaseous neutral calcium is Ar. Calcium atoms have 20 electrons and the shell structure is 28. The calcium ion Ca 2 however has two electrons less.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electron configuration of calcium in ground state by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






