Your Electron configuration of potassium ion images are available in this site. Electron configuration of potassium ion are a topic that is being searched for and liked by netizens now. You can Find and Download the Electron configuration of potassium ion files here. Find and Download all royalty-free photos and vectors.
If you’re looking for electron configuration of potassium ion pictures information related to the electron configuration of potassium ion keyword, you have visit the right site. Our site always gives you suggestions for refferencing the highest quality video and picture content, please kindly surf and find more enlightening video articles and graphics that match your interests.
Electron Configuration Of Potassium Ion. Hence the electron configuration of potassium k is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ potassium ion is formed by losing an electron. Radon xe6s 2 4f 14 5d 10 6p 6. Ne 3s 2 3p 5. So the electron configuration of potassium will involve 19 electrons.
 Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Electron Configuration Electrons Configuration From pinterest.com
Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Electron Configuration Electrons Configuration From pinterest.com
Choice 2 indicates students added an extra electron instead of removing one electron from K. Hence the electron configuration of potassium k is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ potassium ion is formed by losing an electron. Lets begin this section with the orbital box or the orbital representation diagram for a neutral atom. Potassium is in the 1st group. Possible oxidation states are 5. Fe Fe2 and Fe3 Electron.
When we write the configuration well put all 26 electrons in orbitals around the nucleus of the Iron atom.
Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. Draw the electronic configuration for potassium using the electron configuration diagram below. In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Electron Configuration Of K Is 1S² 2S² 2P⁶ 3S² 3P⁶. Its atomic number is 19. This is same as the electron configuration of ar which has 18 electrons.
 Source: in.pinterest.com
Source: in.pinterest.com
If you add the superscripts for this particular atom you. Hence the electron configuration of potassium K is. Hence the electron configuration of potassium k is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ potassium ion is formed by losing an electron. Potassium as an ion exists as K that means it tends to lose an electron as it needs at attain stable electronic configuration and octet valance shell structure resembling that of noble gases. In a neutral atom the number of protons is equal to the number of electrons.
 Source: pinterest.com
Source: pinterest.com
I Answer– the. 68 Compare the radius of a potassium ion to the radius of a potassium atom. Its atomic number is 19. In a neutral atom the number of protons is equal to the number of electrons. Possible oxidation states are 5.
 Source: pinterest.com
Source: pinterest.com
Possible oxidation states are 5. Once we have the configuration for Fe the ions are simple. Electron Configuration Of Potassium Ion. In a neutral atom the number of protons is equal to the number of electrons. 3d Nitrogen Doped Framework Carbon For High Performance Potassium Ion Hybrid Capacitor Sciencedirect.
 Source: pinterest.com
Source: pinterest.com
Electron configuration of K is 1s² 2s² 2p⁶ 3s² 3p⁶. Hence the electron configuration of potassium k is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ potassium ion is formed by losing an electron. What element is best represented by the electron configuration 1s 2 2s 2 2p 6. Draw the electronic configuration for potassium using the electron configuration diagram below. 68 Compare the radius of a potassium ion to the radius of a potassium atom.
 Source: pinterest.com
Source: pinterest.com
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d105p6 Correct Answer. The electron configuration for potassium in the ground state 1s2 2s2 p6 3s2 3p6 4s1 potassium is s block alkali metal with atomic number 19. 68 Compare the radius of a potassium ion to the radius of a potassium atom. Choice 2 indicates students added an extra electron instead of removing one electron from K. So an antimony atom with charge 2 has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1.
 Source: pinterest.com
Source: pinterest.com
Ne 3s 2 3p 5. Potassium K for instance is an important element in all body cells. Potassium as an ion exists as K that means it tends to lose an electron as it needs at attain stable electronic configuration and octet valance shell structure resembling that of noble gases. Electron Configuration Of K Is 1S² 2S² 2P⁶ 3S² 3P⁶. The next six electrons will go in the 2p orbital.
 Source: pinterest.com
Source: pinterest.com
In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. 66 Identify two types of chemical bonding in the source of dietary potassium in this cereal. Write the electron configuration of this potassium ion. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d105p6 Correct Answer. Potassium ion is formed by losing an electron.
 Source: pinterest.com
Source: pinterest.com
That is the first shell of potassium K has two electrons the second shell has eight electrons the 3rd shell has eight electrons and the 4 th shell last orbit has one electron. Fe Fe2 and Fe3 Electron. So an antimony atom with charge 2 has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1. Also you can write potassium electron configuration in abbreviated form ie. In writing the electron configuration for potassium the first two electrons will go in the 1s orbital.
 Source: pinterest.com
Source: pinterest.com
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. Hence the electron configuration of potassium K is. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. What is the electron configuration of the anion I.
 Source: pinterest.com
Source: pinterest.com
Study the third the charges on the chlorine potassium and calcium ions result from a strong tendency of valence electrons to adopt the stable configuration of. 68 Compare the radius of a potassium ion to the radius of a potassium atom. Electron Configuration Of Potassium Ion. Potassium as an ion exists as K that means it tends to lose an electron as it needs at attain stable electronic configuration and octet valance shell structure resembling that of noble gases. It has just one electron in its valence shell.
 Source: pinterest.com
Source: pinterest.com
That is the first shell of potassium K has two electrons the second shell has eight electrons the 3rd shell has eight electrons and the 4 th shell last orbit has one electron. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Electron Configuration Of Potassium Ion. I Answer– the. Study the third the charges on the chlorine potassium and calcium ions result from a strong tendency of valence electrons to adopt the stable configuration of.
 Source: pinterest.com
Source: pinterest.com
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. Then the number of electrons 19 - 1 18. This is same as the electron configuration of ar which has 18 electrons. Study the third the charges on the chlorine potassium and calcium ions result from a strong tendency of valence electrons to adopt the stable configuration of. Draw the electronic configuration for potassium using the electron configuration diagram below.
 Source: pinterest.com
Source: pinterest.com
Once we have the configuration for Fe the ions are simple. Potassium is in the 1st group. Electron configuration of K is 1s² 2s² 2p⁶ 3s² 3p⁶. The electron configuration for potassium in the ground state 1s2 2s2 p6 3s2 3p6 4s1 potassium is s block alkali metal with atomic number 19. Hence the electron configuration of potassium k is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ potassium ion is formed by losing an electron.
 Source: in.pinterest.com
Source: in.pinterest.com
Higher energy the electron configuration for chlorine is written as 1s2 2s2 2p6 3s2 3p5. It has only 1 valence electron. In writing the electron configuration for potassium the first two electrons will go in the 1s orbital. Oct 29 2019 - In this video we will write the electron configuration for K the Potassium ion. Well also look at why Potassium forms a 1 ion and how the electron config.
 Source: pinterest.com
Source: pinterest.com
When we write the configuration well put all 26 electrons in orbitals around the nucleus of the Iron atom. In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom there are 26 electrons. Well also look at why Potassium forms a 1 ion and how the electron config. 3d Nitrogen Doped Framework Carbon For High Performance Potassium Ion Hybrid Capacitor Sciencedirect. Also you can write potassium electron configuration in abbreviated form ie.
 Source: pinterest.com
Source: pinterest.com
Higher energy the electron configuration for chlorine is written as 1s2 2s2 2p6 3s2 3p5. 66 Identify two types of chemical bonding in the source of dietary potassium in this cereal. When we write the configuration well put all 26 electrons in orbitals around the nucleus of the Iron atom. 68 Compare the radius of a potassium ion to the radius of a potassium atom. This is same as the electron configuration of ar which has 18 electrons.
 Source: pinterest.com
Source: pinterest.com
Radon xe6s 2 4f 14 5d 10 6p 6. Remember that potassium is element number 19 so has 19 electrons. Lets begin this section with the orbital box or the orbital representation diagram for a neutral atom. Electronic configuration of Potassium K Potassium with atomic weight 39098 and atomic number 19 has the fundamental electronic configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Its atomic number is 19.
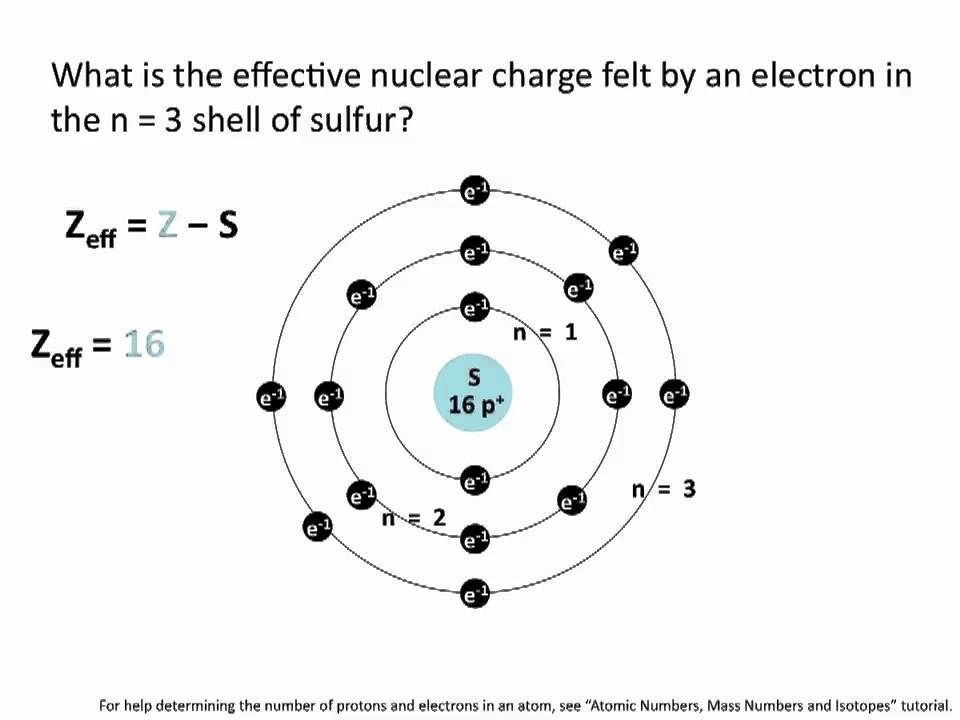 Source: pinterest.com
Source: pinterest.com
Remember that potassium is element number 19 so has 19 electrons. Well also look at why Potassium forms a 1 ion and how the electron config. Electronic configuration of Potassium K Potassium with atomic weight 39098 and atomic number 19 has the fundamental electronic configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Lets begin this section with the orbital box or the orbital representation diagram for a neutral atom. Then the number of electrons 19 - 1 18.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electron configuration of potassium ion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






